Pressmeddelande -
Scandinavian Biopharma announces great progress in the development of ETVAX® as well as a strong organic growth for the distribution business
Scandinavian Biopharma can look back on a very successful 2018! The company was granted 7,4 million Euro from the European and Developing Countries Clinical Trials Partnership (EDCTP) for testing the protective efficacy of the ETEC vaccine candidate ETVAX® in children below five years of age in Low- and Middle-Income Countries. The distribution business achieved a very strong organic sales growth of 58% and the company and its CEO Björn Sjöstrand was recognized with “The entrepreneur of the year in Solna” award.
“To us the grant from EDCTP is another strong proof and recognition of our research. The grant will be used to speed up the clinical development program in Low- and Middle-Income Countries with the ultimate goal to develop a vaccine that has the potential to protect children against life threatening diarrheal disease caused by ETEC”, says Nils Carlin CSO, Scandinavian Biopharma.
The key focus for 2018 was to complete the randomized, double-blinded, placebo-controlled Phase IIB trial in 743 Finnish travellers to Benin in West Africa. The aim of the study is to evaluate safety, new diagnostic methodology and estimate the protective efficacy of ETVAX® against travellers’ diarrhoea (TD) caused by ETEC. ETEC is the most common cause of TD. TD isaffecting 35 million travellers to (sub) tropical destinations every year.
During the end of 2018, the preparations for the pediatric clinical trials in Zambia and the Gambia were initiated. The study in Zambia is estimated to start in the second quarter of 2019 and the large phase IIB in the Gambia will follow in 2020. Scandinavian Biopharma has conducted five successful clinical trials with ETVAX® in the US, Bangladesh and Sweden.
Scandinavian Biopharma continued to expand its organisation during the year by key recruitments in manufacturing and clinical research.
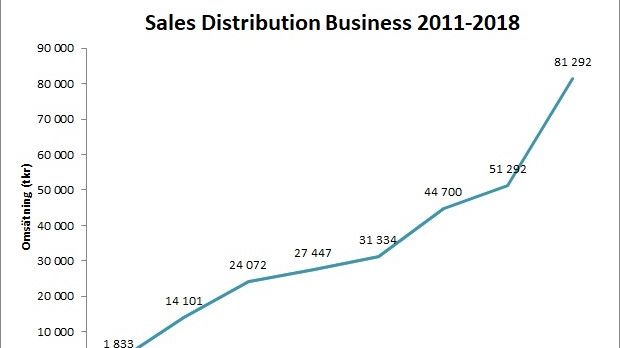
During last year, the company hit a new sales record for the distribution business. The sales increased from 51,3 MSEK in 2017 to 81,3 MSEK in 2018, representing an increase of 58%!
Scandinavian Biopharma has demonstrated strong organic sales growth since the company was founded in late 2009 and the Group has been profitable since 2013.
”The positive progress of the distribution business continued throughout the year of 2018. The increase in growth proved the company’s strategy, to focus on specialty biopharmaceuticals, to be successful”, says Björn Sjöstrand.
In 2018, Scandinavian Biopharma once again won the Swedish national public tender for the booster vaccination dose against diphtheria, tetanus and pertussis (dTp) within the Swedish Immunisation Program for Children. All adolescents in school years 8-9 will thus continue to be offered diTekiBooster for the upcoming years.
During the beginning of 2019 the Ministry of Health in Zambia together with the Swedish Ambassador Henrik Cederin for Zambia announced the start of the company’s trial program in Africa. The company also announced the last follow up visit in the phase IIB clinical trial with ETVAX® in Finnish travellers to Benin. All samples from the clinical trial are now being analysed and later this year the study will be unblinded and presented. In parallel, the program for children in Low- and Middle-Income Countries will progress.
“It’s really exciting times! It feels great to work in a company that truly can make a difference”, Björn Sjöstrand concludes.
Ämnen
Kategorier
Scandinavian Biopharma
We are a Swedish specialty biopharma company developing the first vaccine for protection against diarrhoea caused by ETECin both travellers and endemic populations. The vaccine development project is funded by PATH and Horizon 2020.
Along with the vaccine development, we are experts in marketing and sales of specialty biopharma products in Europe.








