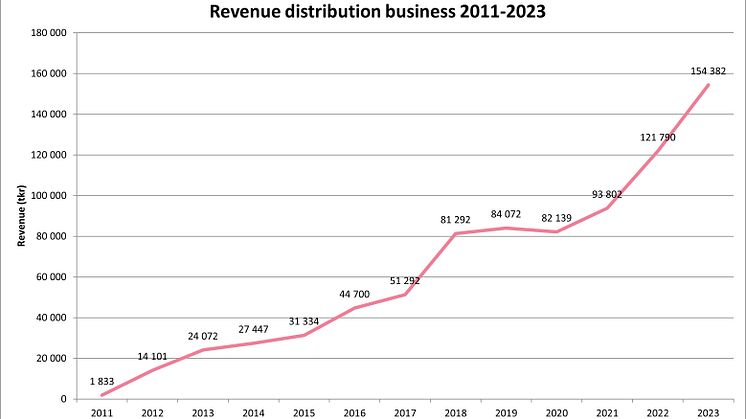
Scandinavian Biopharma reports 27% growth for 2023 and initiates a pre-study ahead of phase III
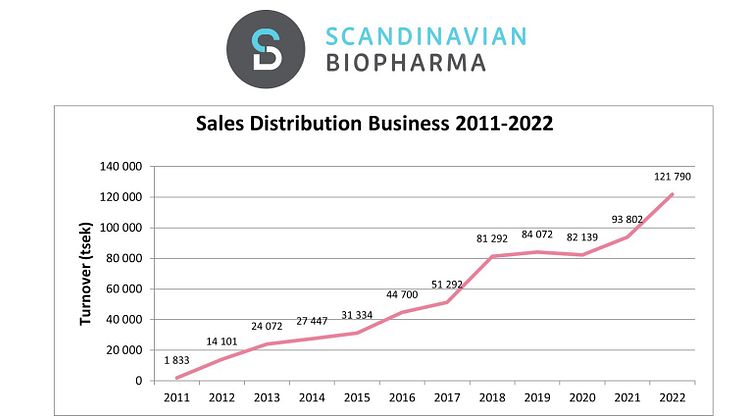
Scandinavian Biopharma is pleased to announce another year of significant growth. For the full year 2023, the revenue increased by over 33 MSEK, corresponding an organic growth of 27%. The total revenue for 2023 amounted to 154 MSEK.