Pressmeddelande -
Scandinavian Biopharma is once again looking back on a very successful business year
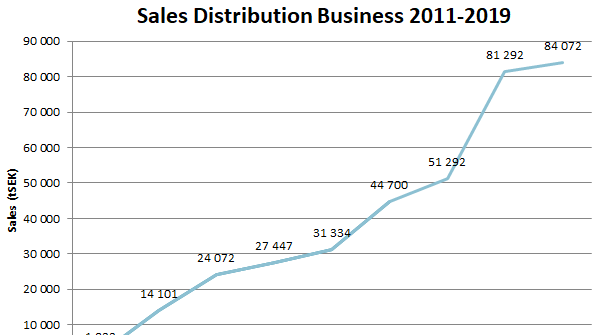
Scandinavian Biopharma can look back on a very successful 2019! The company was granted 10.6 MEUR from the EDCTP for a phase III trial of the ETEC vaccine candidate ETVAX® in children in Low- and Middle-Income Countries (LMIC). Impressive clinical results from Phase I/II study in Bangladesh were published. A late phase African paediatric development program was initiated. The distribution business continued to grow, reaching new record of 84.1 MSEK (7.9 MEUR) 2019.
The grant from EDCTP will accelerate Scandinavian Biopharma’s development program for an all-in-one vaccine formulation that will be tailor-made for the paediatric population in LMIC.
During 2019 the successful results of the Phase I/II study in Dhaka, Bangladesh, were published in The Lancet Infectious Diseases. All predefined primary endpoints for the study were achieved and exceeded, showing that the vaccine was safe and broadly immunogenic, stimulating immune responses to all key vaccine components. “The promising results have a strong clinical relevance and highlight the importance of an effective ETEC vaccine for infants and children in LMICs who are most at-risk of morbidity and mortality due to ETEC” says Nils Carlin, CSO of Scandinavian Biopharma.
A key focus for 2019 has been to initiate a late phase African paediatric development program. In April a Phase I trial in Zambia, including 250 participants down to 6 months of age, started to evaluate the optimal dose of ETVAX® and the benefits of a booster dose in young children. The Zambian study will be followed by a Phase IIb study in approximately 5 000 children in The Gambia. The objectives for the African paediatric development program are to further test safety, immunogenicity and to estimate protective efficacy of ETVAX®. The outcome will pave the way for investments in a phase III program and a pre-qualification by World Health Organization (WHO).
The company was awarded and qualified the honourable title Gazelle Company by the newspaper Dagens Industri. This means that Scandinavian Biopharma belongs to the fastest growing companies in Sweden that are financially profitable with strong financial position.
The Covid-19 pandemic is expected to negatively impact our distribution business, due to changed priorities in the health care system. We still expect to achieve last year’s sales thanks our broader product portfolio. Furthermore, Covid-19 will impact our clinical programme for the same reason and all travel restrictions will effectively delay activities. An important upcoming event during 2020 is the unblinding of the randomized, double-blinded, placebo-controlled Phase IIB trial in 743 Finnish travellers to Benin in West Africa in March/April. Let us hope that all activities that are implemented all over the world will successfully fight the Covid-19 pandemic.
Ämnen
Kategorier
Scandinavian Biopharma
We are a research-based specialty biopharma company determined to give people worldwide a longer and better life. We are developing the first vaccine for protection against diarrhoea caused by ETEC in both travellers and endemic populations. We distribute a wide range of specialty biopharma products with focus on vaccines and immunoglobulins.