
Ribo Completes Recruitment for Phase 2a Trial of our FXI-targeting siRNA Drug
Ribo Completes Recruitment for Phase 2a Trial of our FXI-targeting siRNA Drug

Ribo Completes Recruitment for Phase 2a Trial of our FXI-targeting siRNA Drug
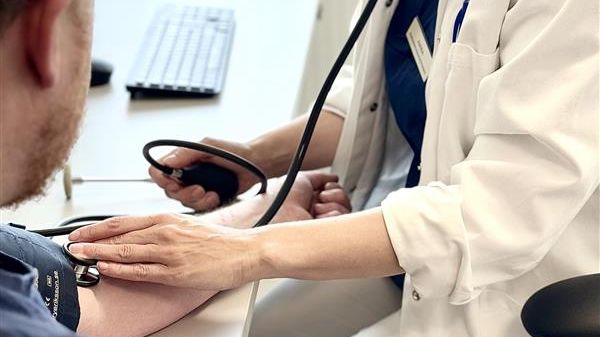
Kliniska studier kommer till vårdcentraler: Unikt samarbete bidrar till utvecklingen av morgondagens läkemedel

Advancing siRNA Drug Discovery and Development with AI
Ribo Advances in Cardiovascular Care with FXI Inhibiting siRNA

First milestone successfully achieved in Ribo’s collaboration on liver diseases with Boehringer Ingelheim
Ribo's siRNA asset RBD5044 receives Clinical Trial Authorization from Swedish MPA for a Phase II trial in patients with mixed dyslipidemia.

Ribo and Pheiron enter strategic collaboration for AI-assisted drug development of RNA therapeutics
Ribo announces authorization to start a Phase I clinical trial in Sweden for siRNA drug RBD7007 targeting Complement Factor 5, C5
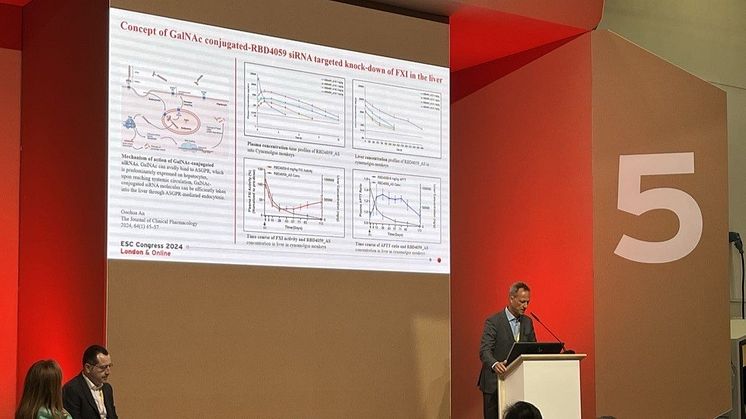
Ribo presented the first clinical data from our completed Ph1 study of the world´s first and most advanced hemostasis-sparing antithrombotic Factor XI (FXI) targeting siRNA asset, RBD4059, based on our RIBO-GalSTAR™ technology.

Ribocure Pharmaceuticals AB announces authorization to start a Phase II clinical trial in Sweden for the worlds first siRNA drug targeting Factor XI: RBD4059

Ribo Receives Granted Patents for Phase 2 HBV siRNA Asset RBD1016 in United States and Europe
Ribo & Ribocure successfully completed enrolment and dosing in the Phase 1 clinical trial, with world’s first siRNA drug targeting coagulation factor XI (FXI)
Vetenskapens Gränd 11, våning 5
431 53 Mölndal
Sverige